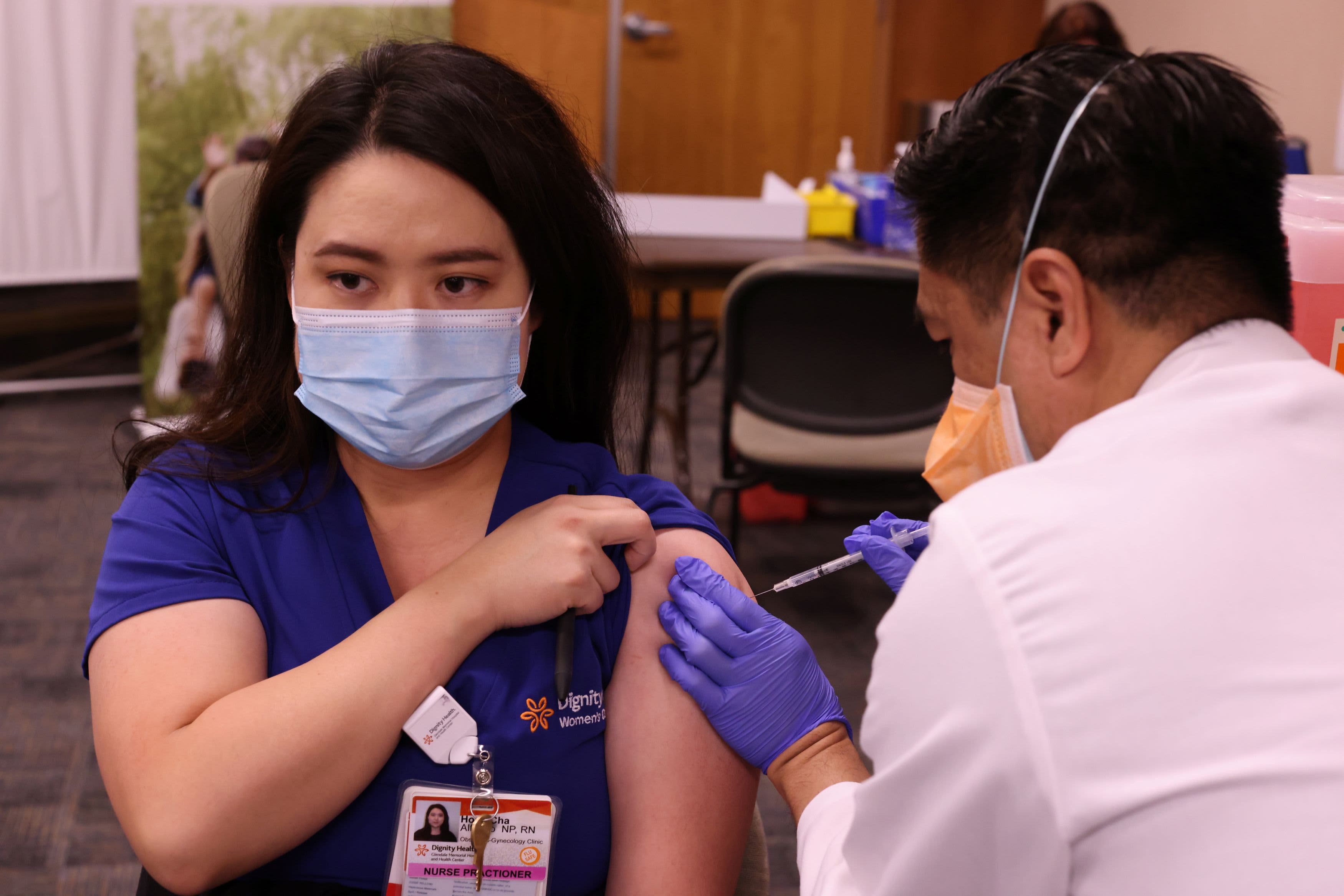
The US is investigating why a handful of people have experienced severe allergic reactions shortly after receiving Pfizer’s coronavirus vaccination shots, an official from the National Institute of Allergy and Infectious Diseases told CNBC Monday.
The study – which is still in its early planning stages – is expected to include “several hundred” people with a history of severe allergic reactions, said Alkis Togias, chief of the Allergy, Asthma and Respiratory Biology Division at the NIAID. His department will lead the study, which researchers hope to begin in weeks, although timing is not guaranteed. Although the reactions have been reported by people who received an injection of Pfizer, the study may look at the vaccines made by both Pfizer and Moderna.
Togias said researchers at NIAID, an agency within the National Institutes of Health, became interested in the rare phenomenon after reports that a few people had reactions to the Pfizer vaccine that qualified as anaphylaxis, a serious and potentially life-threatening allergic reaction. Last week, a doctor in Alaska suffered anaphylactic symptoms about 10 minutes after he received the Pfizer vaccine, making him the third health professional in the state to experience an adverse reaction to the new drug.
“We are a little concerned that people who have had many allergies who have reacted to all kinds of things, not just vaccines, may now be afraid of getting vaccinated,” Togias told CNBC. “We just don’t want that to happen. We want to find a way to get them vaccinated,” he added.
President Donald Trump’s czar against the coronavirus, Moncef Slaoui, mentioned the study during an Operation Warp Speed briefing earlier Monday.
“There is now advanced planning for a study in highly allergic individuals in clinical trials to test the Moderna and Pfizer vaccines and try to understand the immune mechanisms underlying any responses,” he said.
The study comes as the federal government begins to distribute nearly 8 million doses of Covid vaccine across the country this week, after 2.9 million doses of Pfizers vaccine were shipped last week. The US shipped 5.9 million doses of Moderna’s vaccine and 2 million doses of Pfizer’s vaccine this week, Secretary of Health and Human Services Alex Azar said Monday. According to the Centers for Disease Control and Prevention, 556,208 Americans have been shot since Sunday.
It’s unclear why some people get allergic reactions to taking the photos.
Both Pfizer and Moderna vaccines use messenger RNA or mRNA technology. It is a new approach to vaccines that uses genetic material to elicit an immune response against the virus. US health officials say the vaccines are safe, with only 10% to 15% of the volunteers in the clinical trials reporting side effects that were “significantly noticeable.”
Fatigue, headaches, and muscle aches are the most common side effects of Moderna’s vaccine, along with some rare symptoms such as persistent nausea or vomiting and facial swelling likely caused by the shots, according to the Food and Drug Administration. Some of the side effects were difficult to shake, although most resolved within a week, the FDA said.
Medical experts say allergic reactions from vaccines are rare, but can sometimes occur. Still, the FDA said Thursday that it was investigating allergic reactions that occurred after people received the vaccine from Pfizer. Doran Fink, deputy director of the FDA division of vaccines and related product applications, said the agency will consider whether additional recommendations on the vaccines are needed after the investigation.
“At this point, we don’t have enough data to somehow make a definitive recommendation,” he told the Advisory Committee on Vaccines and Related Biologicals at a meeting.
Togias said he hopes the NIAID study will shed some light on the allergic reactions. He said the study may include people who don’t suffer from allergic reactions, so researchers can make comparisons.
Before researchers can begin the study, the agency will have to come up with a very detailed protocol that must be approved by the FDA, Togias said. After it gets an OK from the FDA, it must be reviewed and approved by an ethics committee.
“Of course, when everyone hears a study involving the vaccine, we try to be sensitive and act quickly,” he said. “But it’s not something we can design today and start tomorrow.”