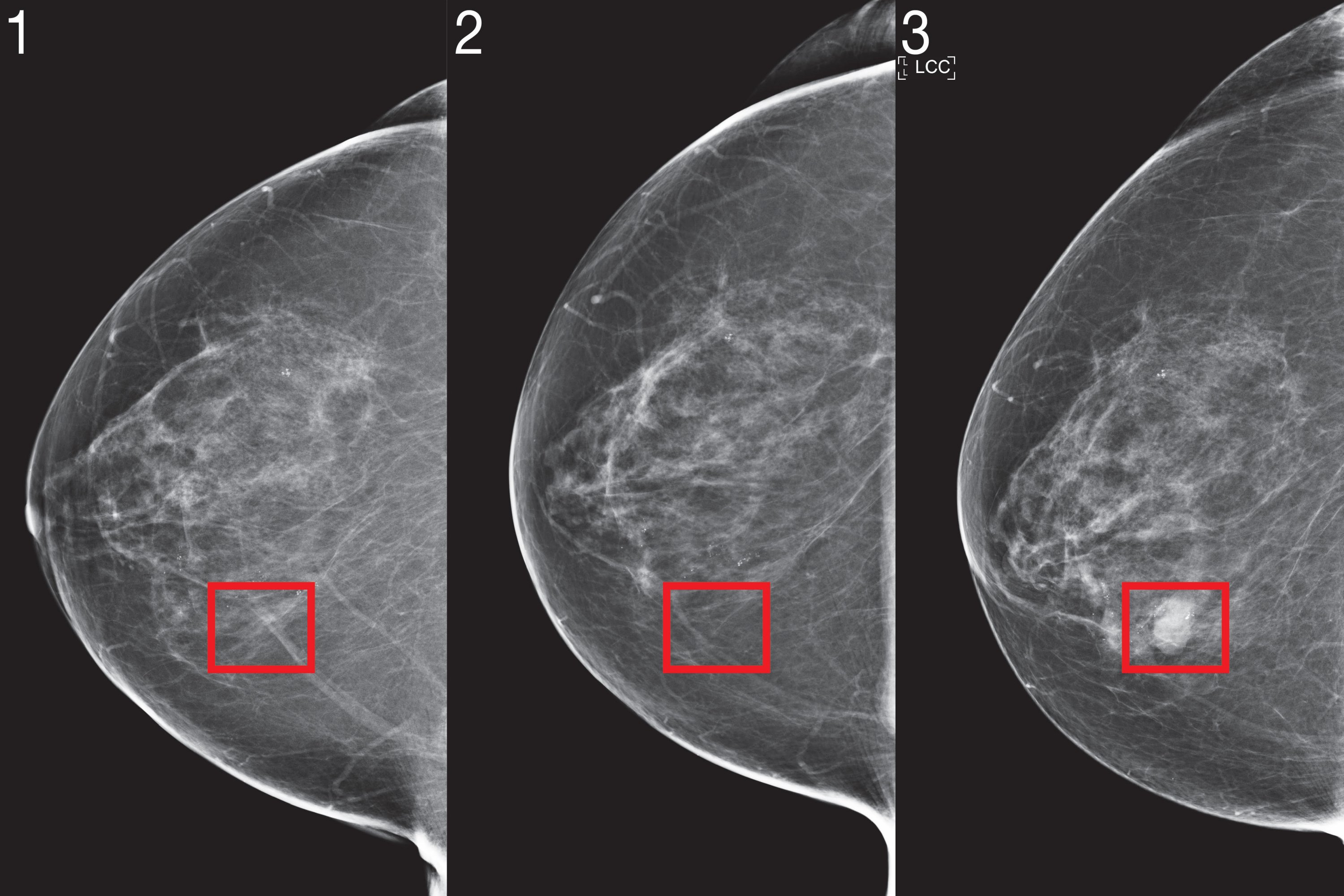
To catch cancer earlier, we have to predict who will get it in the future. The complex nature of risk forecasting is enhanced by artificial intelligence (AI) tools, but the adoption of AI in medicine has been limited by poor performance in emerging patient populations and neglect of racial minorities.
Two years ago, a team of scientists from MIT’s Computer Science and Artificial Intelligence Laboratory (CSAIL) and Jameel Clinic (J-Clinic) demonstrated a deep learning system to predict cancer risk using just a patient’s mammogram. The model showed promising and even improved inclusivity: it was equally accurate for both white and black women, which is especially important given that black women are 43 percent more likely to die from breast cancer.
But in order to integrate image-based risk models into clinical care and make them widely available, the researchers say the models need both algorithmic improvements and large-scale validation across hospitals to prove their robustness.
To this end, they have tailored their new “Mirai” algorithm to the unique requirements of risk modeling. Mirai collectively models a patient’s risk at multiple future time points and can optionally take advantage of clinical risk factors such as age or family history, if available. The algorithm is also designed to make predictions that are consistent across small variations in clinical settings, such as the choice of a mammography machine.
The team trained Mirai on the same dataset from more than 200,000 Massachusetts General Hospital (MGH) exams from their previous work, and validated on test kits from MGH, the Karolinska Institute in Sweden, and Chang Gung Memorial Hospital in Taiwan. Mirai is now installed at MGH and the team members are actively working on integrating the model into healthcare.
Mirai was significantly more accurate than previous methods in predicting cancer risk and identifying high-risk groups in all three datasets. When comparing high-risk cohorts on the HGH test set, the team found that their model identified nearly twice as many future cancer diagnoses as compared to the current clinical standard, the Tyrer-Cuzick model. Mirai was equally accurate for patients of different races, age groups, and breast density categories in the HGH test set and for different cancer subtypes in the Karolinska test set.
“Improved breast cancer risk models allow targeted screening strategies that detect earlier and produce less screening damage than existing guidelines,” said Adam Yala, CSAIL PhD student and lead author of a paper on Mirai published this week in Science Translational Medicine. “Our goal is to make these claims part of the care standard. We are working with clinicians from Novant Health in North Carolina, Emory in Georgia, Maccabi in Israel, TecSalud in Mexico, Apollo in India and Barretos in Brazil to further validate the model on different populations and to investigate how best to do it clinically be implemented. ”
How it works
Despite the widespread acceptance of breast cancer screening, the researchers say the practice is fraught with controversy: more aggressive screening strategies aim to maximize the benefits of early detection, while less frequent screenings aim to reduce false positives, anxiety, and costs to them. who will never even get breast cancer.
Current clinical guidelines use risk modeling to determine which patients should be recommended for additional imaging and MRI. Some guidelines use age-only risk models to determine if and how often a woman should be screened; others combine multiple factors related to age, hormones, genetics and breast density to determine further testing. Despite decades of effort, the accuracy of risk models used in clinical practice remains modest.
Recently, in-depth mammography-based risk models have shown promising performance. To bring this technology to the clinic, the team identified three innovations that they believe are critical to risk modeling: collaborative modeling of time, the optional use of no-image risk factors, and methods to ensure consistent performance in clinical settings.
1 time
Inherent in risk modeling is learning from patients with different amounts of follow-up and estimating risks at different times: this can determine how often they are screened, whether they should undergo additional imaging or even consider preventive treatments.
While it is possible to train individual models to assess risk for each time point, this approach can result in risk assessments that do not make sense, such as predicting that a patient is at higher risk of developing cancer within two years than within five years. . To address this, the team designed their model to predict risks at all times simultaneously, using a tool called an “additive hazard layer.”
The additive hazard layer works like this: their network predicts a patient’s risk at a point in time, say five years, as an extension of the risk at the previous point in time, say four years. By doing this, their model can learn from data with variable amounts of follow-up, and then produce self-consistent risk assessments.
2. Non-image risk factors
While this method focuses primarily on mammograms, the team also wanted to use non-image risk factors, such as age and hormonal factors, if they were available but not needed at the time of the test. One possible approach would be to add these factors as input to the model with the image, but this design would prevent most hospitals (such as Karolinska and CGMH) that do not have this infrastructure from using the model.
In order for Mirai to take advantage of risk factors without needing them, the network predicts that information during training, and if it isn’t there, it can use its own predictive version. Mammograms are rich sources of health information, and so many traditional risk factors, such as age and menopausal status, can be easily predicted from their imaging. As a result of this design, the same model could be used by every clinic worldwide, and if they have that additional information, they can use it.
3. Consistent performance in clinical settings
In order to include in-depth risk modeling in clinical guidelines, the models must perform consistently in different clinical settings, and the predictions cannot be influenced by small variations, such as which machine the mammogram was taken on. Even in a single hospital, the scientists found that standard training did not provide consistent predictions before and after a change in mammography machines, because the algorithm could learn to rely on different signals specific to the environment. To reduce the model, the team used one hostile scheme where the model specifically learns mammogram representations that are immutable with the clinical source environment, to produce consistent predictions.
To further test these updates in a variety of clinical settings, the scientists evaluated Mirai on new test kits from Karolinska in Sweden and Chang Gung Memorial Hospital in Taiwan, and found that it achieved consistent performance. The team also analyzed the model’s performance across races, ages, and breast density categories in the HGH test set and across cancer subtypes in the Karolinska dataset, and found that it performed similarly across all subgroups.
“African American women continue to develop breast cancer at a younger age, and often in later stages,” said Salewai Oseni, a breast surgeon at Massachusetts General Hospital who was not involved in the work. “This, coupled with the higher rate of triple negative breast cancers in this group, has resulted in increased breast cancer mortality. This study demonstrates the development of a risk model whose prediction is remarkably accurate across the race. The likelihood for clinical use is high. ”
Here’s how Mirai works:
1. The mammogram image is run through something called an “image coding program”.
2. Each image view, as well as the view it came from, is merged with other images from other views to produce a view of the full mammogram.
3. The mammogram predicts a patient’s traditional risk factors using a Tyrer-Cuzick model (age, weight, hormonal factors). If not available, predicted values are used.
4. With this information, the additive hazard layer predicts a patient’s risk for each year over the next five years.
Improve Mirai
Although the current model does not look at the patient’s previous imaging results, changes in imaging over time contain a wealth of information. In the future, the team wants to develop methods that can effectively use a patient’s entire imaging history.
In a similar way, the team notes that the model can be further improved by using “tomosynthesis,” an X-ray technique for screening asymptomatic cancer patients. In addition to improving accuracy, additional research is needed to determine how image-based risk models can be adapted to different mammography devices with limited data.
“We know that MRI can catch cancer earlier than mammography, and that earlier detection improves outcomes for the patient,” says Yala. “But for patients with a low risk of cancer, the risk of false positives may outweigh the benefits. With improved risk models, we can design more nuanced risk screening guidelines that provide more sensitive screening, such as MRI, to patients who will develop cancer, to get better outcomes and reduce unnecessary screening and overtreatment for the rest. ”
“We are both excited and humbled to ask if this AI system will work for African American populations,” said Judy Gichoya, MD, MS and assistant professor of Interventional Radiology and Informatics at Emory University, who was not involved at work. “We are studying this question extensively and how we can detect errors.”
Yala co-wrote the article on Mirai with MIT research specialist Peter G. Mikhael, radiologist Fredrik Strand of Karolinska University Hospital, Gigin Lin of Chang Gung Memorial Hospital, associate professor Kevin Smith of KTH Royal Institute of Technology, Professor Yung-Liang Wan of Chang Gung University, Leslie Lamb of MGH, Kevin Hughes of MGH, senior author and Harvard Medical School professor Constance Lehman of MGH, and senior author and MIT Professor Regina Barzilay.
The work was supported by grants from Susan G Komen, Breast Cancer Research Foundation, Quanta Computing, and the MIT Jameel Clinic. It was also supported by Chang Gung Medical Foundation Grant, and by Stockholm Läns Landsting HMT Grant.