
Getty Images
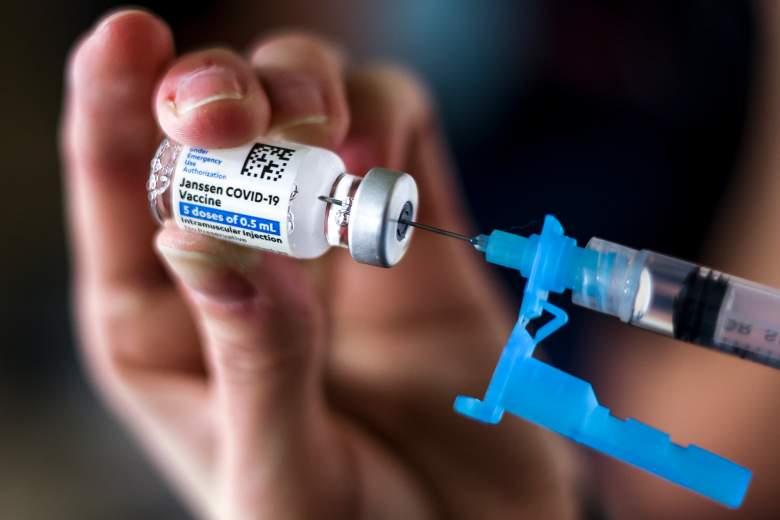
Tiffany Karschamroon takes a dose from a vial of Johnson & Johnson COVID-19, a vaccine approved by the US FDA.
According to information from Reuters news agency, the United States Food and Drug Administration (FDA) stopped production of the vaccine against the virus of Sars-Cov-2, from Johnson & Johnson, at a plant of the biopharmaceutical company Emergent BioSolutions, while an error has which led to millions of doses of the drug being spoiled last March.
The US regulatory authorities ordered production of the vaccine to be stopped #Covid-19 discount #Johnson& Johnson in a facility that previously reportedly damaged 15 million doses of the drug. https://t.co/P5Ay26EQtm
– @ diario24horas (@ diario24horas) April 20, 2021
According to Emergent BioSolutions, the FDA authorities began a new inspection of its facilities in Bayview, Baltimore, on April 12, and last Friday, at the request of that agency, “ the company agreed not to begin production of any new material at the Bayview facility and quarantine existing material manufactured in the Bayview facility until the inspection and disposal of a resulting find is completed, ”said The Washington Post newspaper.
An error in the production of the vaccine
Johnson & Johnson vaccine production was halted at US facility where millions of drug doses were accidentally spoiled – RT https://t.co/CR8tAY3i9o
– Walter Goobar (@wgoobar) April 20, 2021
The crisis of major human error was unleashed on March 31 at a Baltimore facility in a facility owned by Emergent BioSolutions, manufacturer of injectables for AstraZeneca and Johnson & Johnson. The fact that production of this vaccine is now being discontinued was that workers at the factory accidentally combined the ingredients of both drugs, so that about 15 million doses of the Johnson & Johnson vaccine showed manufacturing defects. Likewise, the severity was found to be that one of the ingredients used to make the vaccine had failed quality controls.

The ingredients used in the manufacture of another vaccine, AstraZeneca’s, are said to have been mixed with those of the Johnson & Johnson vaccine, according to The New York Times newspaper, quoted by Diario Las Americas.
This incident cast doubt on Johnson & Johnson’s ability to deliver on its promise, as not only was this amount of vaccines ruined, but authorities shut down the plant as long as it was investigated what had happened.
It should be noted that the quality control detected the faulty doses of the Janssen vaccine, so that these doses never left the production plant, and for its part, the production of AstraZeneca biological products was withdrawn from the plant.
“Human errors are happening,” apologized Dr. Anthony Fauci, the country’s chief epidemiologist. Its antidote to the anxiety it unleashes is precisely that it has been discovered, “there are quality controls for that.” The fact that nothing is left of that plant is another guarantee to him that the system will work. In addition, the Federal Food and Drug Administration (FDA) had not yet approved the plant. The ones administered so far come from the Netherlands, where production was concentrated, according to the Post.
After catching the first bug, Emergent said a single batch of the drug component had been isolated and discarded on April 1. “While it is disappointing to dispose of a batch of pharmaceutical substance in bulk, it occasionally occurs during vaccine production, which is a complex, multi-step biological process,” said La Republica.
“Johnson & Johnson is taking full responsibility” for the incident, the US company said in a statement Saturday, stating that it is “working closely” with the US Food and Drug Administration (FDA) to obtain emergency approval for the production of its vaccine in the United States. Baltimore. plant, according to Diario Las Américas.
In addition, J&J said it “adds dedicated quality and operations leaders and significantly increases the number of technical, quality and manufacturing staff to partner with business specialists already working at Emergent,” the publication said.
Blood Clots: The Johnson & Johnson Vaccine Problem
A CDC advisory committee meets today to discuss how to use Johnson & Johnson’s Covid-19 vaccine in the future following reports of rare but serious cases of blood clots #WSJWhatsNow pic.twitter.com/s93VecUEZM
– The Wall Street Journal (@WSJ) April 14, 2021
On the other hand, although this vaccine had the great advantage of requiring only one dose, its administration was interrupted by US regulators while the study reports on cerebral blood clot formation in humans receiving the injection of this vaccine were revised . Reuters reported.
The cases observed are “extremely rare,” but “the safety of the COVID-19 virus vaccine is a priority for the federal government,” they affirmed, underlining that the recommendation of a pause in administration was decided as a precaution. “Until the process is complete, we recommend a break in the use of this vaccine as a precaution,” said Dr. Anne Schuchat, CDC senior deputy director, and Dr. Peter Marks, director of the evaluation center, in a joint statement. . and FDA biological research.
Finally, citing the discontinuation of production of its vaccines, the Johnson & Johnson pharmaceutical company said it will work closely with Emergent and the FDA to address any findings at the end of the inspection.
The Janssen (Johnson & Johnson) vaccine had been administered to 6.8 million people in the United States when it was suspended a week ago to investigate the link with rare cases of thrombi https://t.co/zTudHcborO
– elDiario.es (@eldiarioes) April 20, 2021
“At this point in time, it is premature to speculate about the potential impact this could have on the delivery of our vaccines,” said the company, which plans to deliver 100 million doses of its vaccine in the first half of 2021. USA to deliver. ver Johnson & Johnson has delivered approximately 18 million doses.
#Watch l The European Medicines Agency said the side effects of the Johnson & Johnson vaccine should be listed on the label after finding a possible link between the biologic and blood clot formation in older adults pic.twitter.com/66kl23aCue
– La República newspaper (@larepublica_co) April 20, 2021
READ MORE: COVID-19: Alaska to vaccinate tourists. Since when?