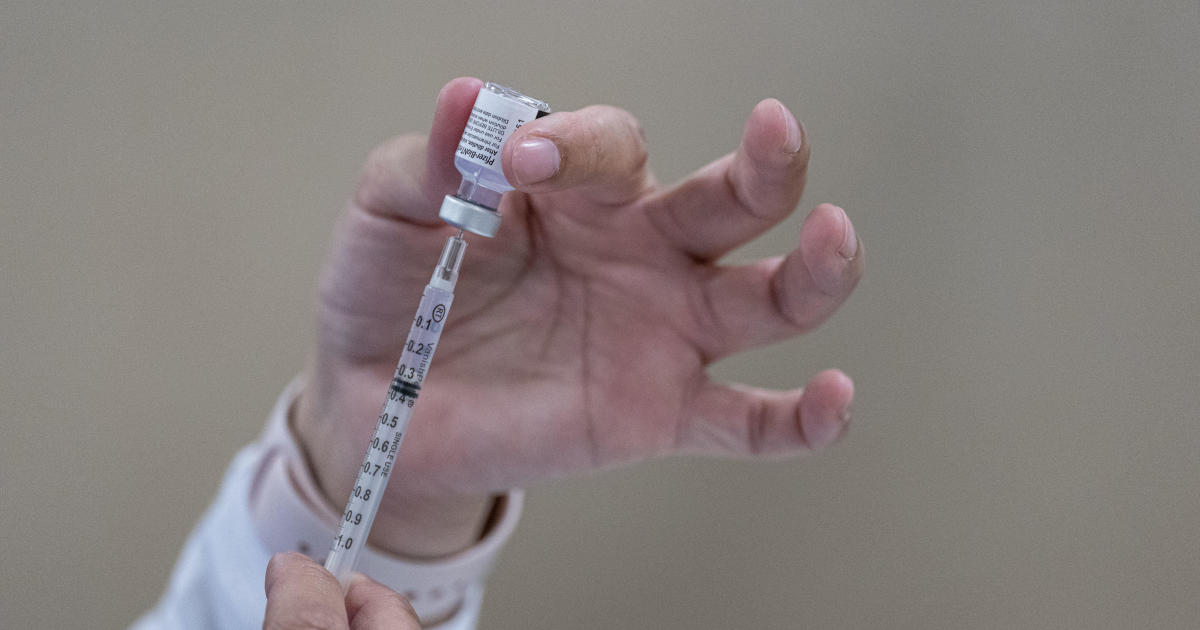
Jerusalem – The first dose of the Pfizer vaccination is 85% effective against coronavirus infection between two and four weeks after inoculation, according to a study published in the medical journal Lancet. The pharmaceutical giant and its German partner BioNTech, meanwhile, have told the U.S. Food and Drug Administration that their vaccine can be safely stored at standard freezing temperatures, which, if approved by the FDA, could facilitate faster distribution due to the need for expensive frozen storage.
The Israeli survey was conducted among health workers at the country’s largest hospital, which launched a massive vaccination campaign on December 19 that is considered the fastest in the world.
Have Israeli studies found the Pfizer vaccine 95% effective a week after a second shot, while the Lancet report focused on more than 9,000 medical personnel at Sheba Hospital near Tel Aviv. About 7,000 of them received the first dose and the rest were not vaccinated.
Of the group, 170 were diagnosed with COVID-19 after tests performed only on those who showed symptoms or who had been in contact with carriers of the coronavirus. Fifty-two percent of them turned out not to have been vaccinated. By comparing the two groups, the Sheba study calculated that the vaccine was 47% effective between one and 14 days after inoculation, increasing to 85% after 15 to 28 days.
“What we are seeing is a really high effectiveness as early as two weeks, between two weeks to four weeks after vaccination, already a high efficacy of 85% reduction in symptomatic infection,” said Gili Regev-Yochay, study co-author. a small group of journalists.
He said that despite the vaccine being “astonishingly effective,” scientists are still investigating whether fully vaccinated people can transmit the virus to others.
“That’s the big, big question. We’re working on it. It’s not on this paper and I hope we’ll have good news soon,” said Regev-Yochay.
So far, research data from only one major vaccine in use worldwide, the Oxford University / AstraZeneca Injection developed in the UK, shows efficacy in preventing asymptomatic infection, suggesting can also help curb transmission
Pfizer says deep freezing is not necessary
Pfizer and BioNTech announced in a joint statement on Friday that they have submitted study data on storage temperatures to the FDA.
The FDA emergency use authorization for the vaccine, issued months ago, requires it to be stored at temperatures well below zero (-112ºF to ‑76ºF), requiring special equipment for both transportation and storage in healthcare facilities. According to the companies’ new recommendation, the vaccine could be stored for up to two weeks at standard freezing temperatures of -13 ° F to 5 ° F, “as an alternative to or complementary to ultra-low-temperature freezer storage.”
“We have continuously conducted stability studies to support commercial-scale production of the vaccine, with the goal of making the vaccine as accessible as possible to healthcare professionals and people in the US and around the world,” said Pfizer CEO Albert Bourla in the Friday’s statement announcing a request to the FDA to change its guidelines. “We appreciate our continued collaboration with the FDA and CDC as we work to ensure that our vaccine can be shipped and stored under increasingly flexible conditions. If approved, this new storage option would provide pharmacies and vaccination centers with greater flexibility in managing their vaccine offer. “
“The data submitted could facilitate handling of our vaccine in pharmacies and provide vaccination centers with even greater flexibility,” added BioNTech CEO and co-founder Ugur Sahin. “We will continue to use our expertise to develop potential new formulations that will make our vaccine even easier to transport and use.”
The other vaccine that has been approved for use and is already widely circulating in the US, made by Moderna, also currently requires freezer storage and transportation according to FDA guidelines for use. The Oxford / AstraZeneca vaccine only requires refrigeration at standard temperatures, making it much easier to move and store, but that shot has not yet received a green light for use in the US.