Dragons lurk at the edges of the map of known elements – atomic giants so delicate and so rare that they defy simple study.
One such colossus has finally revealed at least some of its secrets, with chemists managing to collect just enough einsteinium to work out important details about the mysterious element’s chemistry and its ability to form bonds.
For most of 70 years, einsteinium isotopes have proven frustratingly difficult to study. Either they are far too difficult to make, or they have a half-life of less than a year, and the precious little that is created starts to disintegrate at high tide like a sandcastle.
The behavior of the element is believed to follow the patterns of its less robust colleagues in the actinide series. That is obvious. But because of its sheer size, strange relativistic effects make it more difficult to predict how it will react in certain chemical processes.
Usually, such confusion is easily resolved by simply running a series of experiments.
The Lawrence Berkeley National Laboratory at the U.S. Department of Energy has finally gathered enough stuff to do just that.
More informally referred to as the Berkeley Lab, the famous institute is already responsible for the discovery of a significant portion of the upper limits of the periodic table of elements.
A dozen of these were the work of nuclear physicist Albert Ghiorso, a lifelong Berkeley researcher whose early career saw him develop radiation detectors as part of the Manhattan Project.
In the early 1950s, Ghiorso discovered faint traces of two as yet unidentified radioactive elements in airborne dust collected by planes flying through the aftermath of the first full test of a thermonuclear device.
One of those elements was later called einsteinium, named after none other than the famous German-born theorist himself.
With an atomic mass of 252 and no less than 99 protons, it is not a lightweight. As with all transuranic elements – elements heavier than uranium – einsteinium requires some serious physics to produce.
There is no convenient resource or supply to dive into. To prepare a batch, smaller family members, such as curium, have to be shot with a bunch of neutrons in a nuclear reactor, and then you have to be very patient.
Early efforts in the 1960s yielded just enough to see with the naked eye, weighing just 10 nanograms. Subsequent attempts succeeded somewhat better, although they usually resulted in impure batches.
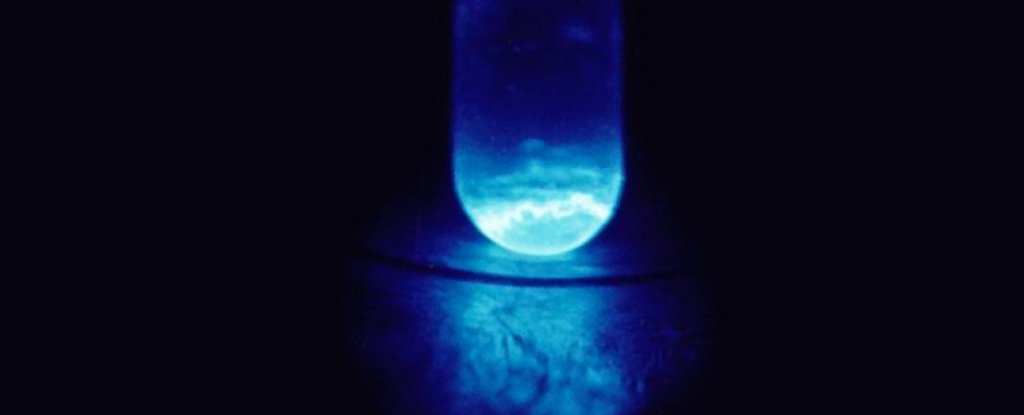
This time, researchers came up with about 200 nanograms of the einsteinium isotope E-254, framed as part of a complex with a carbon-based molecule called hydroxypyridinone.
Getting this far was not easy, marred by contamination of smaller elements and then the inevitable consequences of mid-pandemic shutdown – exactly what would threaten an experiment that relies on rapidly decomposing material.
“It is a remarkable achievement that we have been able to work with this small amount of material and do inorganic chemistry,” says researcher Rebecca Abergel.
“It’s important because the more we understand about the chemical behavior, the more we can apply this understanding to the development of new materials or new technologies, not necessarily just with einsteinium, but also with the rest of the actinides. in the periodic table. “
Subjecting their disappearing stack of chelated E-254 atoms to X-ray absorption tests and photophysical measurements revealed important details about the element’s bonding distance, while also demonstrating wavelength-shifting emission behaviors not seen in other actinides.
Einsteinium is right on the brink of what we can achieve using benchwork chemistry. While larger elements exist, their increasing size places them beyond the reach of current technology’s ability to create enough for analysis.
But the more we learn about heavy atoms like einsteinium, the more likely we are to find stepping stones to build giants that are really off the map somewhere.
“Similar to the newest elements discovered in the last 10 years, such as tennessine, which used a berkelium target, if you could isolate enough pure einsteinium to make a target, you could look for other elements and get closer to the (theoretical) island of stability, ”says Abergel.
This research is published in Nature.