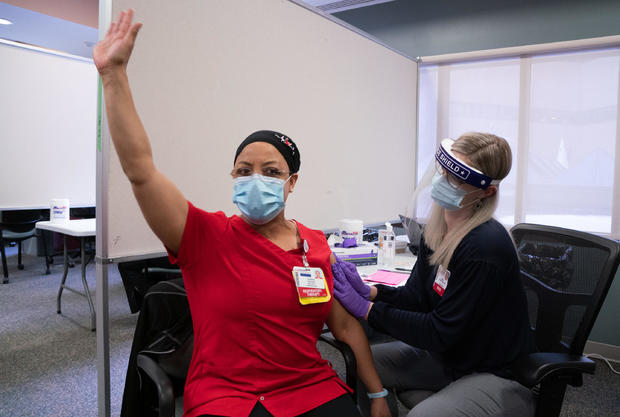
Hundreds of U.S. hospitals will begin vaccinating their employees Tuesday, while federal health officials are reviewing a second COVID-19 vaccine needed to boost the country’s largest vaccination campaign. Packed in dry ice to keep at ultra-frozen temperatures, shipments of Pfizer’s COVID-19 vaccine to 400 additional hospitals and other distribution locations will be shipped one day after the The nation’s death toll surpassed a whopping 300,000.
The Food and Drug Administration will publish its analysis Tuesday of a second thoroughly studied COVID-19 vaccine that could soon join Pfizer-BioNTech in the fight against the pandemic. If FDA advisers give it a positive recommendation on Thursday, the agency may give the green light to drug manufacturer Moderna’s vaccine later this week.
A second vaccine cannot come soon enough. The virus’s devastating toll is only expected to increase in the coming weeks, fueled by leisure travel, family reunions and lax adherence to basic public health measures.
Because the vaccine requires two rounds, the people getting injections now will need a second injection in about three weeks.
The first 3 million shots are strictly rationed to primary care workers and elderly care patients, and hundreds of millions of shots will be needed over the next few months to protect most Americans. Recordings for nursing home residents will not begin until next Monday in most states, when some 1,100 facilities will begin vaccinations.
The federal government coordinates mass deliveries by private shipping and distribution companies based on locations chosen by the state’s governors.
After another initial batch of deliveries Wednesday, officials from the Trump administration’s Operation Warp Speed in Washington said they will move 580 additional shipments this weekend.
“We’ll start our drumbeat of continuous implementation of the vaccine as soon as it becomes available,” Army General Gustave Perna, chief operating officer for Warp Speed, told reporters Monday. “We package and deliver. It’s a constant stream of available vaccines.”
Perna and other US officials reiterated their forecast that 20 million Americans will be able to get their first shots by the end of December, and another 30 million in January.
That projection presupposes swift approval of the vaccine under review this week, co-developed by Moderna and the National Institutes of Health. Like the Pfizer BioNTech vaccine, Moderna’s requires two injections for complete protection.
Last month, Moderna and NIH reported that their shot was found to be nearly 95% effective for different ages and racial groups, according to the results of an ongoing study involving 30,000 people. The main side effects were fatigue, muscle pain and pain at the injection site after the second dose. Those flu-like reactions are common with many vaccines and are a sign that the vaccine is triggering the immune system to fight the virus.
Moderna did not report any major safety concerns from its research. But the FDA panel will certainly examine the data for evidence of possible serious allergic reactions or other rare side effects. Being civil servants in the UK researching different side effects there The vaccine from Pfizer and the FDA is closely following the rollout here for similar reports.
The shots from both Moderna and Pfizer-BioNTech are so-called mRNA vaccines, a completely new technology. They are not made with the coronavirus itself, which means that no one can see it from the photos. Instead, the vaccine contains a piece of genetic code that trains the immune system to recognize the enriched protein on the surface of the virus.
The first deliveries of vaccines were an encouragement to exhausted doctors, nurses and hospital workers across the country.
UW Health / John Maniaci
“I feel relief now, I know we have a long way to go, but I can see that light now at the end of the tunnel,” University of Louisville Health nurse LaShawn Scott told CBS News. “I’m saving lives by getting this vaccination.”
Sandra Lindsay of the Long Island Jewish Medical Center in New York got her first injections just after 9 p.m. ET on Monday, making her one of the first in the country to receive the authorized vaccine.
“I hope this is the beginning of the end of this painful time in our history. We are in a pandemic, so we all need to do our part,” she told CBS News.
For Dr. Tina Wu of NYU Langone Health, the vaccine is more than just an injection, it’s a prescription for getting back to normal. “I haven’t hugged my mom for 10 months for fear. Now I can finally do it,” she told CBS News.
Johnnie Peoples, a 43-year-old survival flight nurse, was excited and a bit nervous Monday afternoon when he unzipped his flight suit and extended his left arm to become the first person to receive the vaccine at the University of Michigan Medical Center in Ann Arbor.
“Just being part of it is a good feeling,” he said.
In Florida, government officials expect to have 100,000 doses of the vaccine in five state hospitals by Tuesday.
“This is 20,000 doses of hope,” said John Couris, president and chief executive officer of Tampa General Hospital, following Monday’s delivery of 3,900 vaccine vials. Each vial has five doses.