An understanding of early human development is crucial if we are to improve assisted reproductive technologies and prevent pregnancy loss and birth defects. However, studying early development is challenging – few human embryos are available and research is subject to significant ethical and legal constraints. The emergence of techniques using cultured cells in vitro constructing mammalian embryo models therefore offers exciting possibilities1Two papers in it Nature now make significant progress in this area, showing that human embryonic stem cells2 or cells reprogrammed from adult tissues23 can be induced to self-organize in a shell, forming structures resembling early human embryos. This is the first integrated human embryo model to contain cell types related to all stem cells of the fetus and its supporting tissues.
In mammals, a fertilized egg undergoes a series of cell divisions during the first days of development, leading to the formation of a structure called the blastocyst. The blastocyst contains an outer cell layer called the trophectoderm that surrounds a cavity with a cell cluster called the inner cell mass (ICM). As the blastocyst develops, the ICM separates into two adjacent cell populations – the epiblast and the hypoblast (known as the primitive endoderm in mouse embryos). The blastocyst then implants into the uterine tissue and sets the stage for an event called gastrulation, where epiblast cells give rise to the three basic cell layers that will make up the entire fetus. The trophectoderm then forms most of the placenta and the hypoblast forms some layers of a structure called the yolk sac, which is necessary for early fetal blood supply.
The first in vitro models to recapitulate blastocyst formation using cultured cells (structures known as blastoids) used mouse stem cells that correspond to the stem cells found in the epiblast, trophoblast and primitive endoderm in the mouse blastocyst46However, the generation of similar blastoids from human cells has not been achieved so far1Previous models of early human development used human stem cells similar in development to post-implantation, pre-gastrulation epiblast cells79While as such they were able to recapitulate some of the stages of human development after implantation, they lacked descendants associated with the trophectoderm, hypoblast, or both.
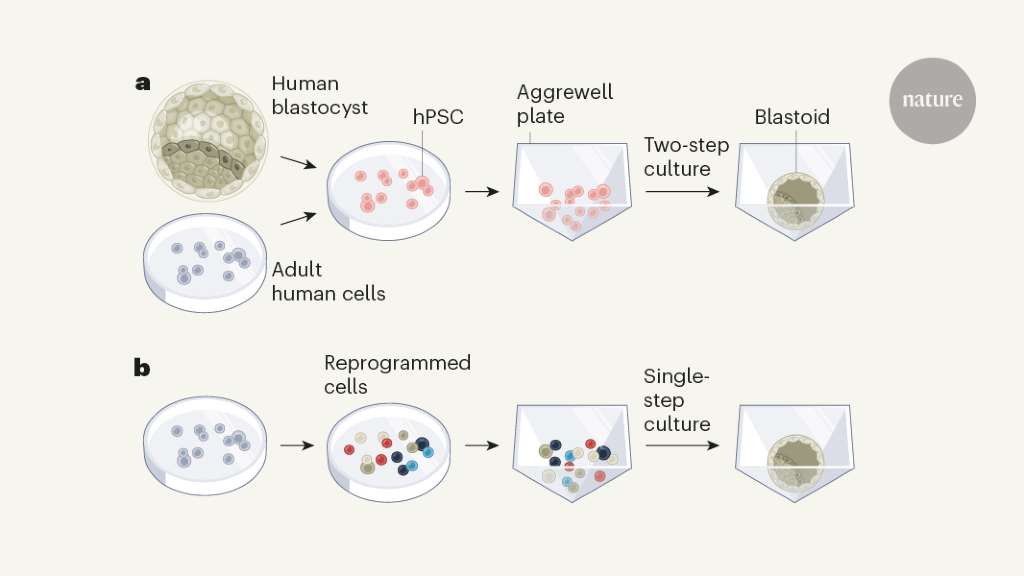
In the current papers, Yu et al2 and Liu et al3 describe human blastoids. The key to these technological breakthroughs appears to have been twofold: first, the use of cells representative of lines in the human blastocyst; and second, optimization of culture protocols.
Yu et alStarted with human embryonic stem cells, which are derived from human blastocysts, or induced pluripotent stem cells, which are generated from adult cells. Importantly, both stem cell types are developmentally similar to epiblast cells in the blastocyst, and may also lead to lineages related to the trophectoderm and hypoblast. Liu on the other hand et alReprogrammed adult skin cells called fibroblasts to form a mixed cell population containing cells with gene expression profiles similar to those of epiblast, trophectoderm and hypoblast cells. Just like in the mouse blastoid protocols46, both approaches involved seeding the cells in 3D culture dishes called Aggrewell plates, and treating them with liquid growth medium containing chemical factors to regulate the signaling activities required for blastocyst development (Figure 1). Yu and colleagues treated the cells in sequence with two different types of culture medium to promote differentiation of the cells into lines representative of the trophectoderm and hypoblast.
Both groups found that human blastoids emerged after 6-8 days of culture, with a formation efficiency of up to nearly 20%, comparable to the efficiency of mice blastoid protocols.46The human blastoids were of a similar size and shape to natural blastocysts, with a similar total number of cells. They contain a cavity and an ICM-like cluster.
Detailed characterization of the blastoids (including genome-wide expression analysis and comparisons with human embryo data) showed that their cell lines show molecular similarities to those of the human blastocyst before implantation. The spatial organization of the epiblast, trophectoderm and hypoblast-related lineages is consistent with that found in preimplantation human embryos. The groups also showed that the blastoid cells have key properties of blastocyst lines – cells isolated from the blastoids could be used to generate different stem cell types. Yu et alShowed that when these stem cells were transplanted into mouse blastocysts, they produced cells that could integrate with the corresponding murine lines in the mouse embryo.
Next, the researchers analyzed the further development of the blastoids using an established test that mimics implantation in the uterus in culture dishes. Like blastocysts, when blastoids were cultured for four to five days in this test, some bound to the culture dish and continued to develop. In a portion of these attached blastoids, the cell line representative of the epiblast was reorganized into a structure enclosing a central cavity – reminiscent of the pro-amniotic cavity that forms in the epiblast of post-implantation blastocysts. And in some blastoids, the trophectoderm-related cell line spread and showed signs of differentiation into specialized placental cell types. Yu et alAlso observed a second cavity in the hypoblast-related cell line in some blastoids, related to the yolk sac.
Together, the data from the groups shows that human blastoids show promise in vitro models of preimplantation and early postimplantation blastocyst development. However, there are notable limitations that must be overcome. For example, the development of the blastoids is inefficient and varies between cell lines produced by different donors and between experimental batches. In addition, the three lines appear to develop at slightly different rates in single blastoids, and blastoid development in the same scale does not appear to be synchronized. The spatial organization of the hypoblast-related lineage in blastoids remains to be improved. In addition, the blastoids contain unidentified cell populations that have no counterparts in natural human blastocysts.
Another challenge is that blastoid development is limited in the post-implantation stages, unlike blastoids in mice.46Further optimization of the culture and experimental conditions is needed to improve the culture of human blastoids after implantation. in vitro, up to the equivalent of 14 days in vivoStrict ethical rules prevent the cultivation of human embryos after this stage, when structures related to gastrulation begin to appear. Three-dimensional systems for culture of human blastocysts10, which effectively promote post-implantation development, could help improve our ability to grow blastoids to this limit, by preserving the normal 3D tissue architecture and spatial relationships between the different cell lines in the blastoids.
Human blastoids are the first human embryo models derived from cultured cells in vitro and which all have stem cells of the fetus and its supporting tissues. As protocols have been optimized, these blastoids will better mimic human blastocysts. This inevitably leads to bioethical questions. What should the ethical status of the human blastoids be and how should they be regulated? Should the 14-day rule apply? These questions must be answered before research on human blastoids can proceed with caution. For many people, the study of human blastoids will be less ethically challenging than the study of natural human blastocysts. However, others might view human blastoid research as a path to human embryo development. The continued development of human embryo models, including human blastoids, thus requires public discussions about the scientific significance of such research, as well as the social and ethical issues it raises.
