As the geneticist Theodosius Dobzhansky said1 in 1973, “Nothing in biology makes sense except in the light of evolution.” Many modern biologists might add that nothing in molecular biology makes sense except in the light of biochemistry – without the quantitative understanding biochemistry provides, how can biologists predict the effect of a two-fold lowering of the levels of a protein during the early development of an organism? , or a tenfold increase in the concentration of another protein in cancer cells? The gap between the streamlined experiments of biochemistry and the messy complexity of the cell seemed unbridgeable for a long time. Now, sign up Nature, Sharma et al.2 report a technique that enables the biochemical analysis of molecular interactions in cells.
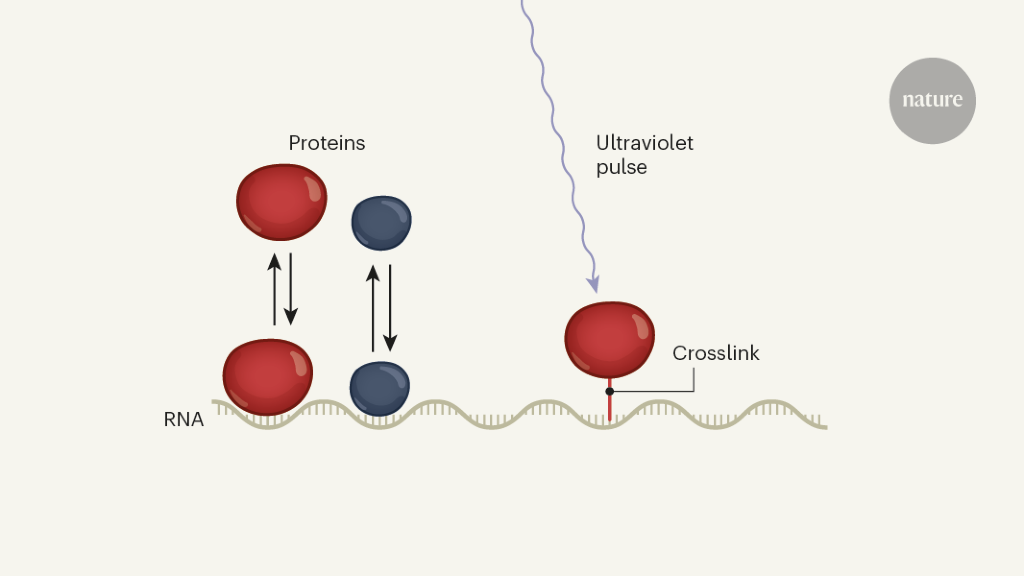
The authors focused on the dynamics of interactions between RNA molecules and proteins. Messenger RNA molecules are bound by several RNA binding proteins (RBPs), which control almost every aspect of the mRNA life cycle – from the initial processing of newly created RNAs to their final destruction3. Each RBP can bind to hundreds of RNA molecules, and in turn, each RNA can be bound by dozens of different RBPs4. In addition, RNA protein interactions are not static5,6. Instead, proteins can quickly bind to and dissociate from their target RNAs just as quickly (Fig. 1), and these dynamics are at the heart of gene regulation. In other words, the kinetics of RNA-protein interactions are a driving force behind gene expression. Defining the parameters of these kinetics in cells is therefore crucial to fully understand the regulation of gene expression.
Although RNA-protein interactions have been studied for decades, their kinetics in cells have not been characterized. Broadly speaking, kinetic insight is only available from in vitro studies with purified proteins; experiments in cells have been able to identify the RNA targets of RBPs, but lack the precision to measure the kinetics of the interactions5. With the advent of high throughput sequencing methods, in vitro approaches can now investigate the kinetics of a protein’s interactions with tens of thousands of RNA variants7. But these experiments are still performed on purified proteins in the absence of the cellular environment. In recent years a method called crosslinking and immunoprecipitation8 (CLIP) has become a workhorse for the characterization of RNA-protein interactions in cells. In CLIP, a protein in complex with an RNA molecule is covalently cross-linked to the RNA using ultraviolet light; the complexes are then isolated and the cross-linked RNA identified by high-throughput sequencing. This approach provides a catalog of RNAs that bind to a specific RBP in the complex environment of the cell, but it only provides a snapshot of these interactions at best.
Sharma and colleagues are now bridging the gap between in vitro strategies and CLIP by developing a type of CLIP that can determine the kinetic parameters of RNA-protein interactions in cells. The authors’ main insight was that certain technical aspects of previously reported CLIP methods prevented such approaches from being useful for capturing kinetic parameters. The most challenging limitation is that the crosslinking rates must be fast to capture the rates at which proteins and RNA molecules associate and dissociate. Conventional UV sources cannot achieve sufficiently fast cross-linking, so using them to measure kinetics is the same as using a slow shutter speed to photograph a galloping horse – everything blurs in the image. This realization led the authors to use a pulsed femtosecond UV laser, which couples proteins to RNA fast enough to record kinetic parameters. They call their method KIN-CLIP (for kinetic CLIP).
To test the method, the authors applied it to an RBP called Dazl, which is required for reproductive cell production and regulates gene expression9. Dazl binds to hundreds of target mRNAs, increasing their stability and the number of proteins produced10. However, despite its biological importance, much about the binding and function of Dazl is unknown, making it an ideal candidate for KIN-CLIP experiments.
Sharma and colleagues first verified that KIN-CLIP identifies RNA targets found in previously published datasets produced with ‘snapshot’ CLIP. They then calculated kinetic parameters, known as rate constants, for the association and dissociation of Dazl with each of its thousands of binding sites in RNA. These results showed that Dazl binding is very dynamic: the binding time is short; the RBP stays at separate locations for only a few seconds. Dazl also rarely binds, which is why the binding sites are free from the protein most of the time.
The authors also found that multiple Dazl molecules tend to bind in places that are close to each other. The kinetic analysis suggests that this may be due to cooperative binding – a phenomenon where the binding of one protein to one site increases the likelihood that other proteins will bind to nearby sites. Finally, the authors incorporated Dazl’s newly determined kinetic parameters into a predictive model of its impact on gene expression, providing a biochemical basis for its function and paving the way for future research.
One of the most exciting aspects of this study is the potential of KIN-CLIP for studying other RBPs, but the method has some limitations. As with all CLIP-based techniques, the ability to cross-link the protein of interest to bound RNAs is required; this can prove challenging, as some proteins do not have the necessary side chains that are well oriented for cross-linking. The biggest hurdle to potential KIN-CLIP conversions, however, is that specialized equipment is required for the cross-linking: pulsed femtosecond lasers may not be easily accessible to many biologists. In addition, the experimental procedures and associated analysis of KIN-CLIP libraries are more complicated than those of standard CLIP experiments, and could prove to be another barrier to adoption.
Nevertheless, this study has brought the tools of biochemistry into living cells and could thus be a turning point in the study of RNA-protein interactions. The next step is to apply KIN-CLIP to other RBPs, but the prospect of applying it to other types of interacting biomolecules is also on the horizon. Indeed, the authors intriguingly note that pulsed femtosecond lasers can cross-link proteins with DNA – perhaps a ‘DNA KIN-CLIP’ is at your fingertips. Sharma and colleagues have not only set a new standard in RNA biology, they may have unleashed the power of biochemistry on molecular biology in general.
