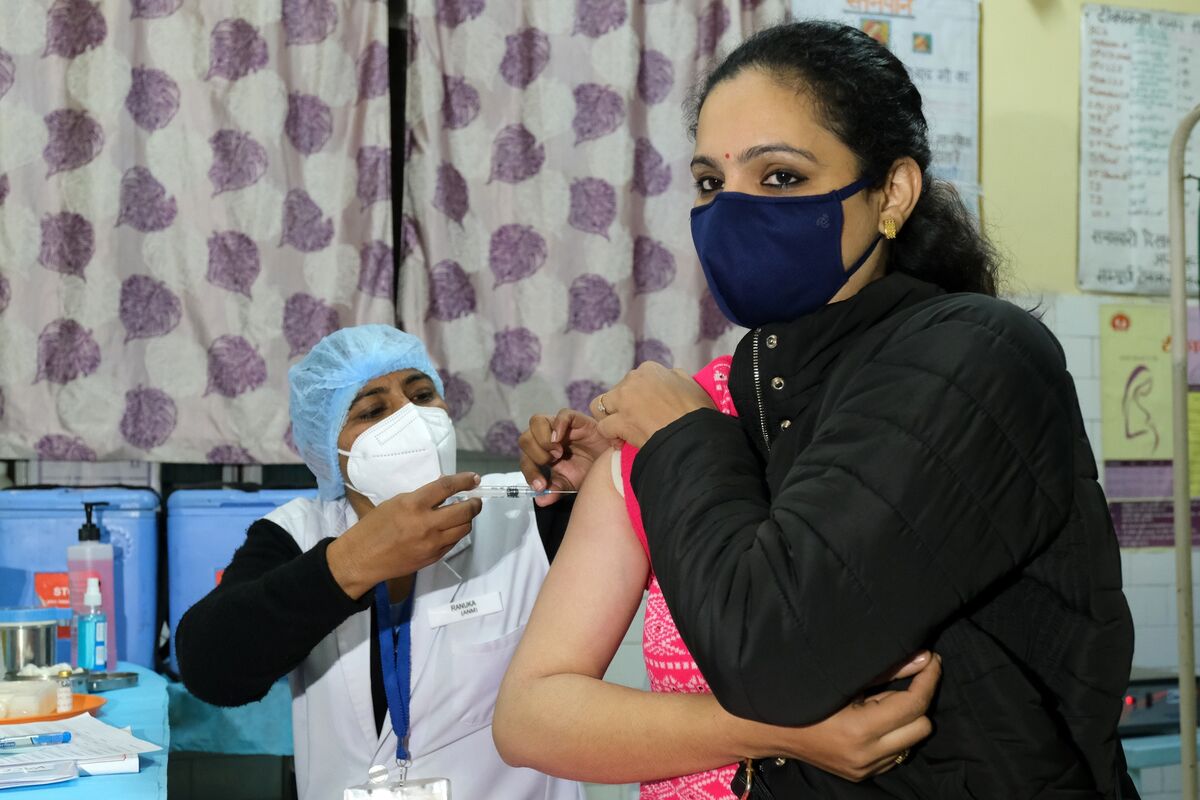

A trial process for vaccine delivery in Delhi, India, on Jan. 2.
Photographer: T. Narayan / Bloomberg
Photographer: T. Narayan / Bloomberg
India has tracked the UK and granted emergency approval for the coronavirus vaccine developed by AstraZeneca Plc and the University of Oxford, the first step in the plan to inoculate civilians in the country where the world’s second largest outbreak of Covid-19 occurs.
Information and Broadcasting Minister Prakash Javdekar said the AstraZeneca recording is locally produced by the Serum Institute of India Ltd. – the world’s largest vaccine producer by volume – was approved Friday.
“India may be the only country where four vaccine candidates are ready.” Javdekar said during the briefing of the ruling Bharatiya Janata party in New Delhi on Saturday. “Yesterday, one vaccine was approved for emergency use, Serum’s Covishield.”
India’s Drugs Controller General has yet to formally announce approval. Serum has an agreement with AstraZeneca to roll out at least a billion doses and has already taken millions of shots. The move came just days after the UK regulator approved the vaccine, which will be rolled out to Britain’s most vulnerable groups starting Monday.
Astra-Oxford Covid Shot gets first approval with UK Nod
The approval means India can begin to vaccinate its population of about 1.3 billion people. This is a difficult task given the country’s vast territory, limited infrastructure and fragmented health networks. The South Asian nation already has more than that 10.2 million confirmed infections and as many as 149,000 deaths.
AstraZeneca’s vaccine, which has the most supply agreements worldwide, has been posited as a more suitable means of reaching people in the remote areas of India’s hinterland than a vaccine developed by Pfizer Inc. and BioNTech SE is also under consideration.
Cold storage
Pfizer’s vaccine requires freezing conditions for transport and storage, while AstraZeneca can be stored at refrigerator temperatures and is also expected to be cheaper.
Still, data from clinical studies indicate that the Astra injection may be less effective than that from Pfizer and another similar vaccine from Moderna Inc., each of which showed 95% efficacy in studies.
The first data from Astra and Oxford in November raised concerns about the extent to which the vaccine would protect. The studies yielded two different results from two dosing regimens. The partners said their vaccine was 90% effective when given half a dose before a full dose booster, and two full doses showed 62% efficacy.
Astra-Oxford vaccine research leaves important questions unanswered
While trial results published in The Lancet found that the vaccine is safe and effective, more analysis will be needed to see how well it works in people over 55, among those at higher risk from the pandemic. A US trial that aims to evaluate the shot at 40,000 people is underway and should clarify some of these questions, with results expected in early 2021.
Local doses
Human trials conducted by Serum in India have also been chased allegations made by a volunteer who claimed to have serious side effects from the vaccine and demanded compensation. Pune-based serum denied the claims, saying the volunteer’s illness had nothing to do with the shot.
Serum has said half of all vaccines it produces will remain in India, with 100 million doses being produced in December for the local vaccination campaign, Chief Executive Officer Adar Poonawalla said in an interview in November.
The Astra vaccine accounts for more than 40% of deliveries to low- and middle-income countries, followed on the basis of agreements by London-based research firm Airfinity Ltd.
– Assisted by Abhijit Roy Chowdhury and Santosh Kumar