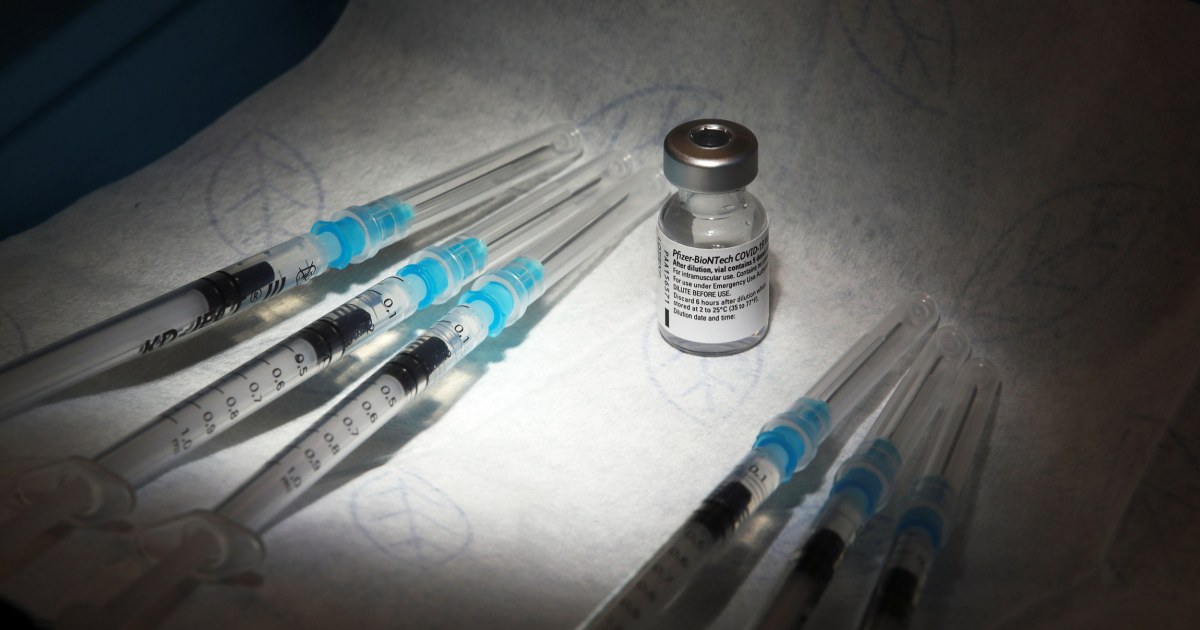
Despite its 95 percent effectiveness in preventing coronavirus infection after two doses of its vaccine, Pfizer now sees what a third dose could do.
The company announced on Thursday that a booster dose is being studied in people who received their first doses of the vaccine more than six months ago.
Full coverage of the coronavirus outbreak
In an interview with NBC News’ Lester Holt, Pfizer CEO Albert Bourla said the hope is that a third dose will boost the immune response even higher, providing better protection against variants.
“We think the third dose,” said Bourla, “will increase the antibody response 10 to 20 fold.”
The new study will monitor the safety and efficacy of a third dose in two age groups: those 18 to 55 and those 65 to 85. The participants are from a group of people who were among the first to receive the Pfizer-BioNTech vaccine: people who volunteered for Pfizer’s first phase 1/2 clinical trial, which began in May.
During that trial, the participants received two doses of the vaccine three weeks apart. The same dose interval is what is currently recommended.
The third shot will be exactly what the participants got a year ago.
Pfizer also plans to test whether a modified version of the vaccine works well against the South African variant.
Indeed, if SARS-CoV-2 changes, the vaccines may need to be adjusted. The Food and Drug Administration issued guidelines on Monday saying that vaccine manufacturers may be able to forgo lengthy clinical trials to prove the safety and effectiveness of vaccines modified to account for variants.
That’s no different from how the flu shot changes from year to year as it explains the strains most likely to infect people.
“You have to get your flu vaccine every year,” Bourla said. “It will be the same with Covid. In a year you will have to get your annual injection to protect Covid.”
That suggests that even if the pandemic ends, Covid-19 could be here to stay. Ongoing studies of redesigned vaccines are needed to understand when boosters may be needed, outside experts said.
“You have to cast a big net to find Goldilocks,” said John Grabenstein, a former director of medical affairs for vaccines at Merck and a former defense department immunologist. “You want to watch at shorter intervals, you want to watch at longer intervals to determine when is the best time to re-vaccinate, if necessary.”
Download the NBC News app for full coverage of the coronavirus outbreakk
So far, evidence suggests that the existing Pfizer-BioNTech vaccine remains effective against variants first identified in the UK, Brazil and South Africa.
Bourla said the company’s goal is to run and adapt the current vaccine within 100 days if and when another variant emerges.
Moderna, which makes a similar Covid-19 vaccine, announced on Wednesday that it has also begun to study the effects of adding a third dose to its regimen and has developed a version of the vaccine designed to treat the variant. from South Africa.
Follow NBC HEALTH on Twitter & Facebook.
