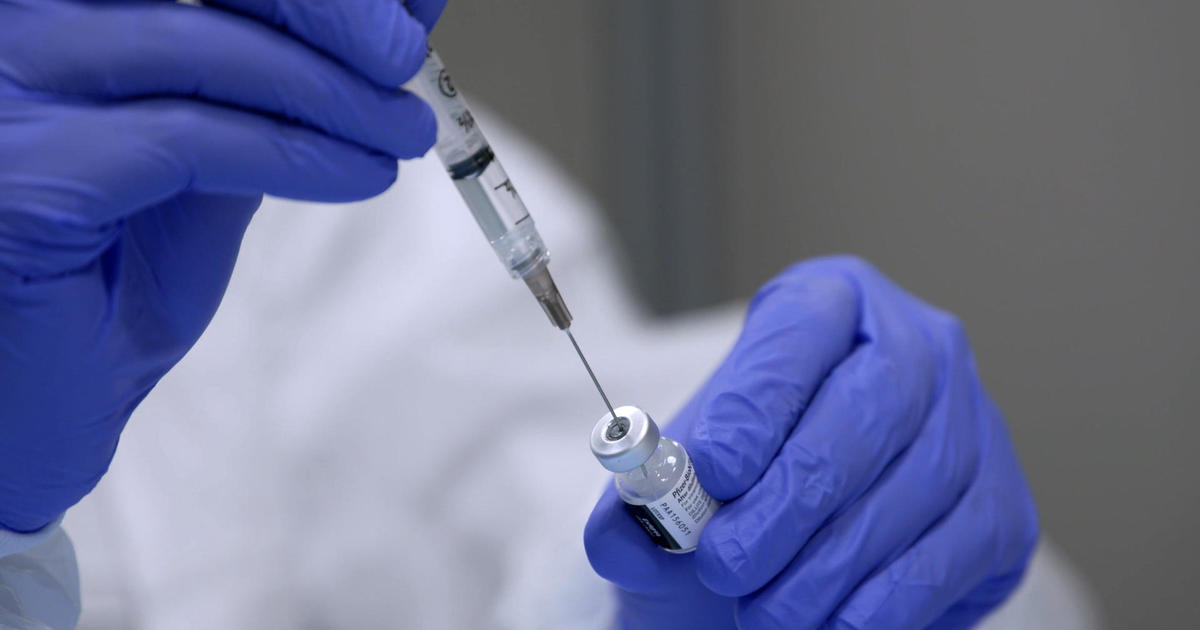
More than 15,000 of our family members, friends and neighbors were affected by the coronavirus in the past week. So we could almost hear a national sigh of relief as the pharmaceutical company, Pfizer, began delivering the first doses of its vaccine nationwide. Pfizer, a frequent advertiser on this broadcast, and its German partner, BioNTech, were the only major vaccine developers to refuse federal funding for research and development, yet they were the first to receive emergency use approval from the FDA. On Friday, the biotech company Moderna was also given permission. As part of Operation Warp Speed, the federal government set a target of having 20 million inoculations by the end of the year. But that goal may be ambitious; the rollout of the vaccine was rocky. Yet for the first time since the pandemic, an end appears to be in sight, thanks to a revolutionary advance in biotechnology.
Bill Whitaker: This is a global pandemic, the worst in a century. A vaccine with this technology had never appeared on the market. That’s a big bet you made that this would work.
Kathrin Jansen: Yeah, I didn’t see it that often – maybe as a bet, because we’re scientists. That’s what we do for a lifetime, every day. We discover new things. Everything is new.
Kathrin Jansen is head of vaccine research and development for Pfizer. Based in New York, it is one of the largest pharmaceutical companies in the world. Jansen, who developed an interest in science as a child in Germany, grew up developing vaccines against pneumonia and the HPV virus. So when she first heard about the new coronavirus, her thoughts immediately turned to a vaccine.
Bill Whitaker: When you started this mission, New York was on fire with this virus.
Kathrin Jansen: Yes. We lived in a hot zone in New York. And we saw firsthand what was happening – every day. And Bill, the most chilling thing to me was when we walked our dog. And you see one refrigerated truck after another appearing in the parking lots in front of the hospitals.
Bill Whitaker: Morgues in refrigerated trucks?
Kathrin Jansen: morgue, right. That definitely fueled the desire to come up with a vaccine, whatever it takes.
Bill Whitaker: This was quite personal?
Kathrin Jansen: I have taken this very personally. I wanted to fight it, beat it, fight it. It was … nothing else mattered.
Across the Atlantic, in Mainz, Germany, doctors Ugur Sahin and Ozlem Tureci also focused on the fight against the coronavirus. Founders of a cutting-edge biotechnology company called BioNTech, were collaborating with Pfizer on a flu vaccine when Sahin read an article about a mysterious illness in Wuhan, China on Jan. 24.
Dr. Ugur Sahin: We knew that most likely we are facing a global pandemic. And we knew we had no time to waste.
Dr. Ozlem Tureci: We started thinking about – how to implement a vaccine development program from the start – and so we had to run the whole business.
Bill Whitaker: Did you have any doubts about that?
Dr. Ugur Sahin: I had no doubts. The only thing that worried me is that we may be late.
The couple’s company, BioNTech, is pioneering vaccines made with mRNA molecules in our cells that pass genetic instructions from our DNA to particles that make proteins, the building blocks of life.
Dr. Ugur Sahin: We felt responsible to develop a vaccine because we knew the potential of our technology.
Manipulating mRNA molecules in the laboratory to fight disease has been considered promising technology for over 30 years, but it has never yielded a proven vaccine. With the coronavirus spreading across Europe, Sahin and Tureci redoubled their efforts. By February, BioNTech had produced 20 different versions of mRNA that elicited immune responses in mice and monkeys. Sahin knew his small business would need help taking his research outside of the lab, so he picked up the phone and called his friend at Pfizer, Kathrin Jansen.
Dr. Ugur Sahin: And she said, “Ugur, why are you calling me?” And I said, “Kathrin, we started making a vaccine against COVID-19. I wanted to ask if you think Pfizer would join us.” And she said, “Of course, Ugur, actually I wanted to call you because we are also interested in developing a vaccine, and it will be our main project.”
It became more of an obsession for Pfizer’s CEO, Albert Bourla …
Albert Bourla: What I thought was if – if – not us, then who would? We have a lot of experience with vaccines. We have a lot of production capacity with vaccines. I went and I said, “We have to do something to see if we can help develop a medical solution.”
Since March, he had been pressuring Pfizer scientists to quickly develop a vaccine. He set a deadline for October.
Albert Bourla: Then – of course, I gave them some tools too. I told them to think: this is not normal practice. Return on investment is not taken into account. This is considered w– to have an open checkbook, which is precisely–
Bill Whitaker: An Open Checkbook.
Albert Bourla: Yes.
Bill Whitaker: Did you have any idea how much you would be willing to spend?
Albert Bourla: Costs us about $ 2 billion. And I knew if we fail and have to write it off, it will be very painful. But it won’t bring Pfizer down.
Kathrin Jansen convinced her boss, Albert Bourla, that mRNA technology had the best chance of meeting his tight deadline. Through her work with BioNTech on a flu vaccine, she was convinced that mRNA was on the verge of a breakthrough. So Pfizer’s CEO, Bourla, signed off on the partnership and rolled the dice on the experimental technology.
Bill Whitaker: There was a good chance you wouldn’t be able to.
Albert Bourla: I believe in the power of science. I believe in the power of the private sector. And I believe in the wonders that private sector science can do for humanity.
Unlike old-school vaccines made with real virus that often grows slowly in eggs, these mRNA molecules are produced quickly in a lab, programmed with some of the virus’s genetic code. We’ve all seen pictures of the coronavirus with its crown of spike proteins. The mRNA vaccine instructs your healthy cells to make replicas of the spikes. They can’t make you sick, but they do teach the immune system what the virus looks like. If the real virus shows up, your immune system’s antibodies will attack.
In May, Pfizer was ready to start testing the vaccine at various locations in the US
Dr. Mark Mulligan: And I raised my hand and said yes.
She tapped Dr. Mark Mulligan, director of the NYU-Langone vaccine center in Manhattan.
Dr. Mark Mulligan: I’ve worked on HIV / AIDS, Zika, Ebola – flu pandemic vaccines. So now is really the time for us to jump in and say, “Okay, let’s do it. Let’s try to be part of the solution.”
Nearly 44,000 people around the world volunteered for the staged, double-blind studies, where the vaccines were tested against a placebo – most were between the ages of 16 and 85. Researchers faced some skepticism when they contacted African American and Hispanic volunteers.
Bill Whitaker: Were these communities well represented in the lawsuits?
Dr. Mark Mulligan: I feel like they were. If you add the Hispanic population and the African American black population and the Native American population, it was just under 40%. We would have liked it a little better, especially with African Americans. We were just under 10%. But I think it’s generally good and we’ve had several town halls with community partners in Harlem. It’s important for us to be able to say, “Yes. That we tested it in your community. Yes, it was just as patient, safe and just as protective.
Typically, phased tests are performed sequentially. To speed up the process, the FDA allowed these studies to be conducted simultaneously. Dr. Mulligan told us he had never seen it happen so quickly.
Bill Whitaker: What do you say to people who are concerned that this was going too fast, that this will be rushed to market?
Dr. Mark Mulligan: Given the massive public health emergency we find ourselves in internationally, it was appropriate that all speed was used. I’ve been doing vaccine trials for 30 years and I … I promise you haven’t cut back on the usual safety assessments.
Blood samples from volunteers were taken to this Pfizer facility in Pearl River, New York, where these robots worked day and night to analyze the effectiveness of the vaccine. They will continue to collect and analyze samples for two years.
Kathrin Jansen: These robots have probably performed more than 180,000 tests.
When the critical results of the third phase were revealed last month, Kathrin Jansen took a break in the country with her husband. She received a call from Pfizer’s CEO, Albert Bourla.
Kathrin Jansen: And I said, “What is it, Albert?” He said, “Well, Kathrin, we talked to the FDA, we can say the vaccine is over 90 percent effective.” And I said, “What? (LAUGH) That’s great.” So my husband and I were right next to me, we jump up and down.
Dr. Mark Mulligan: To have a vaccine that was so badly needed, and to have it 95% protective, and to make it happen so quickly, my experience is second to none.
Bill Whitaker: Can we all just say goodbye to our masks?
Dr. Mark Mulligan: I’m afraid not. We are not yet sure whether the vaccines will prevent asymptomatic infection. The vaccine is unlikely to make a difference to this current wave that we are in.
Bill Whitaker: We will be through this for a while.
Dr. Mark Mulligan: Absolutely. This won’t be like a light switch that is on / off, but rather like those dimmer switches that we slowly turn up to turn the lights on. 5%, 10%, 20% 50% of people are vaccinated for months. But to end the pandemic in 2021, we probably need to get 75% of people vaccinated and then we can stop the pandemic.
There are still some unknowns about this vaccine: how long does it take; are there any long term safety concerns, why have some people had severe allergic reactions? It has yet to be tested on young children and pregnant women. But the most pressing issue: the bumpy rollout. The development of the vaccine was rapid, but so far its distribution has been anything but. Pfizer reduced the expected number of doses for 2020 by more than 50%, citing a shortage of raw materials. States complain they don’t get clear guidelines from the federal government’s Operation Warp Speed about what they get and when. At the moment the light at the end of the tunnel is still dim.
When the US death toll from the pandemic reached 300,000 last week, the bells of the National Cathedral in Washington DC rang 300 times – one in every 1,000 Americans killed by the virus.
Bill Whitaker: This vaccine is called a miracle. How would you describe it?
Kathrin Jansen: We can call it a miracle. But a miracle always feels like it just happened. It didn’t just happen. Turn right? It was something that was intentional. It was done with passion, done with passion. It was urgency. You always had that devastating disease in your sight.
Produced by Marc Lieberman. Associate producer, Ali Rawaf. Broadcaster, Emilio Almonte. Edited by Sean Kelly.



